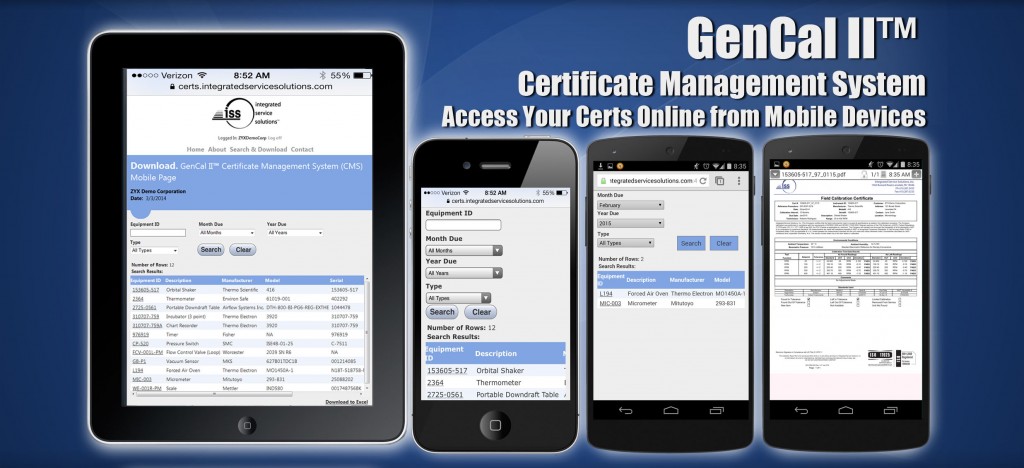
Announcing Browser Applications for Mobile Devices
March 3, 2014— Integrated Service Solutions, Inc. announced today the release of custom designed browser applications allowing customers easy access to view electronic calibration reports on mobile devices.
In February, the Company officially launched their GenCal II™ Certificate Management System. This custom designed database is now optimized for the growing mobile device market.
Catherine Peetros, Director of Operations said, “Earlier this year, our in-house IT group designed an easy-to-use interface and a centralized, searchable database to store, view and retrieve electronic certificates. Now they’ve enhanced this product by adding more flexibility. This latest addition of value added services for our customers includes a browser application allowing them to view their electronic documents on mobile devices such as tablets, the iPhone and Droid devices.”
George Fenzil, Director of IT added, “This system gives an authorized QA representative at a customer site the ability to spot check equipment calibration records with an iPhone or iPad. This convenient and quick access to electronic documents makes it easy to pull up electronic records by scanning the bar coded label. It’s another way to differentiate our services and provide our customers with the latest in technology.”
To ensure the integrity of the data, the platform uses multiple layers of security such as; Secure Socket Layer (SSL) encryption, password protection, a company-owned and managed firewall and other state of the art technologies. Customers request login credentials and all logins and downloads are recorded with a timestamp, user id and IP address.
“Providing our client base with a well organized document management system, ensures that they have ready access to all of their electronic documents in the event of an FDA or client audit and helps reduce administrative overhead for them,” added Peetros.
Integrated Service Solutions, Inc. has been a pioneer in the use of electronic calibration reports and bar coded labels as part of their comprehensive equipment calibration and maintenance program. The company’s electronic documents and e-signatures, introduced more than eight years ago, are 21 CFR 11 Compliant. This compliance is part of the FDA Code of Regulations that defines criteria to ensure that electronic records are trustworthy and reliable.
For more information about the GenCal II Certificate Management System, please email customerservice@integratedservicesolutions.com or click here http://integratedservicesolutions.com/contact/gencal-ii-certificate-management-system-cms/.
Posted in: Blog, Company News
Leave a Comment (0) ↓